By Du Yifei, People's Daily
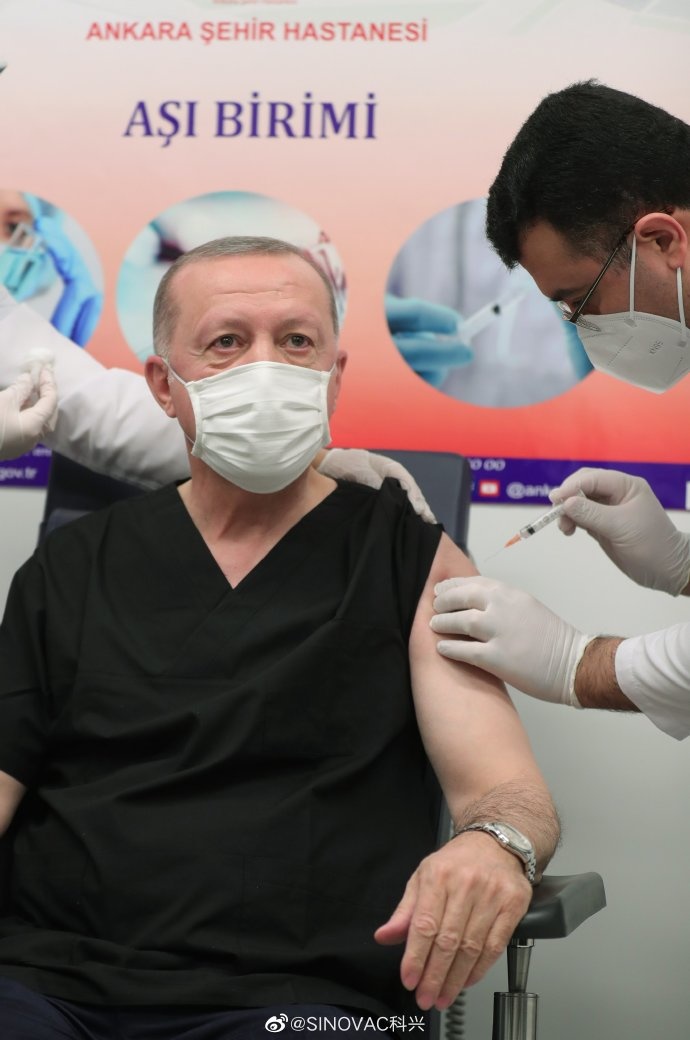
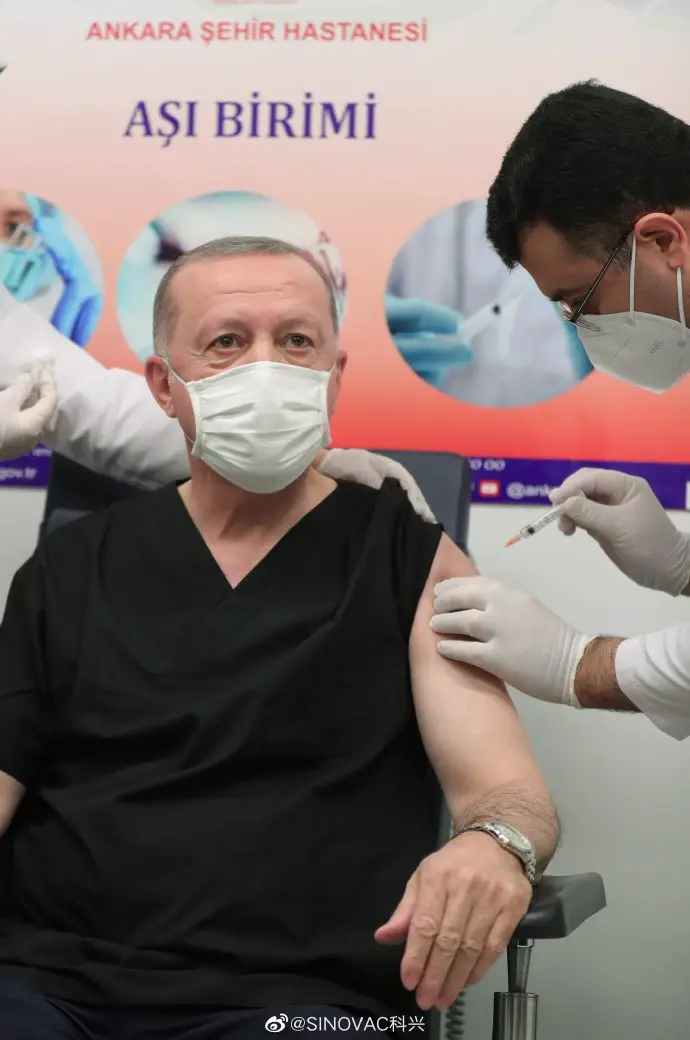
Turkish President Recep Tayyip Erdogan receives the CoronaVac vaccine in Ankara on Jan. 14. He announced his vaccination on social media later. (Photo from Erdogan's Twitter page)
Brazil's Health Regulatory Agency (Anvisa) announced on Jan. 17 that it has approved the emergency use of the CoronaVac vaccine, from the Chinese biotech firm Sinovac. The first batch of Brazilian vaccines were vaccinated on the same day.
This made Brazil the third country in the world to approve the emergency use of the CoronaVac vaccine, after Indonesia and Turkey. Chinese vaccines' overall safety and efficacy are widely being recognized in the world.
The CoronaVac vaccine started Phase I and II clinical trials in last April. After bring proved to be safe and effective, it was put into Phase III clinical trials in Brazil, Turkey, Indonesia and Chile. According to Turkey's interim data, the Chinese vaccine is 91.25 percent effective. It was also proved to have an efficacy rate of 65.3 percent by Indonesia's Phase III clinical trials. Brazil's Phase III clinical trials showed that the vaccine is 100 percent effective against severe symptoms and 78 percent for mild cases, which led to a general efficacy of 50.38 percent.
Yin Weidong, chairman of Sinovac, said on a Jan. 13 press conference that the Phase III clinical studies have already proved the safety and efficacy of the Chinese vaccine in the global context.
"We have a projected annual production capacity of more than 1 billion doses," Yin said, adding that the biotech firm has built a production line that is able to produce about 500 million doses of the CoronaVac vaccine solutions annually. "The production line for the second phase of our vaccine solutions will be put into operation in February this year," he continued.
It is reported that multiple countries, including Indonesia, Turkey, Malaysia, Singapore and the Philippines have signed deals with Sinovac for vaccine supply. The vaccine developed by the company was also injected to multiple state leaders, which demonstrated their trust to the Chinese vaccine.
Turkish President Recep Tayyip Erdogan received his first dose of the CoronaVac vaccine in Ankara on Jan. 14, local time, a day after Turkey announced to approve Sinovac vaccines for emergency use. CoronaVac is the first COVID-19 vaccine approved by the country.
Indonesian President Joko Widodo received the first dose of CoronaVac on Jan. 13, live on television. Indonesia's food and drug authority BPOM issued an emergency use authorization to the vaccine on Jan. 11, explaining the vaccine has undergone a Phase III trial in Bandung, and its efficacy was higher than the 50 percent threshold stipulated by the World Health Organization.
Malaysian state-run pharmaceutical company Pharmaniaga on Jan. 12 reached a deal on COVID-19 vaccine cooperation with Sinovac. The Chinese firm will provide 14 million doses of ready-to-fill COVID-19 vaccines to Malaysia, and the Malaysian company will carry out a fill-and-finish process of the vaccine in Malaysia.
The Ukrainian Lekhim Group also partnered with Sinovac on the purchase of 5 million doses of CoronaVac vaccine. Besides, the Ukrainian firm is planning to produce locally multiple Sinovac vaccines, including the CoronaVac, from 2022.
Brazilian Health Minister Eduardo Pazuello said on Jan. 7 that Brazil has signed an agreement with the Butantan Institute of Sao Paulo to purchase 100 million doses of CoronaVac vaccine.
The Department of Health of the Philippines recently disclosed that the Philippine government has secured 25 million doses of Sinovac-developed CoronaVac vaccine. The country's Food and Drug Administration said it has received an application from the Chinese firm seeking an emergency use authorization for the CoronaVac vaccine. The vaccine is expected to be granted as early as this February.
This made Brazil the third country in the world to approve the emergency use of the CoronaVac vaccine, after Indonesia and Turkey. Chinese vaccines' overall safety and efficacy are widely being recognized in the world.
The CoronaVac vaccine started Phase I and II clinical trials in last April. After bring proved to be safe and effective, it was put into Phase III clinical trials in Brazil, Turkey, Indonesia and Chile. According to Turkey's interim data, the Chinese vaccine is 91.25 percent effective. It was also proved to have an efficacy rate of 65.3 percent by Indonesia's Phase III clinical trials. Brazil's Phase III clinical trials showed that the vaccine is 100 percent effective against severe symptoms and 78 percent for mild cases, which led to a general efficacy of 50.38 percent.
Yin Weidong, chairman of Sinovac, said on a Jan. 13 press conference that the Phase III clinical studies have already proved the safety and efficacy of the Chinese vaccine in the global context.
"We have a projected annual production capacity of more than 1 billion doses," Yin said, adding that the biotech firm has built a production line that is able to produce about 500 million doses of the CoronaVac vaccine solutions annually. "The production line for the second phase of our vaccine solutions will be put into operation in February this year," he continued.
It is reported that multiple countries, including Indonesia, Turkey, Malaysia, Singapore and the Philippines have signed deals with Sinovac for vaccine supply. The vaccine developed by the company was also injected to multiple state leaders, which demonstrated their trust to the Chinese vaccine.
Turkish President Recep Tayyip Erdogan received his first dose of the CoronaVac vaccine in Ankara on Jan. 14, local time, a day after Turkey announced to approve Sinovac vaccines for emergency use. CoronaVac is the first COVID-19 vaccine approved by the country.
Indonesian President Joko Widodo received the first dose of CoronaVac on Jan. 13, live on television. Indonesia's food and drug authority BPOM issued an emergency use authorization to the vaccine on Jan. 11, explaining the vaccine has undergone a Phase III trial in Bandung, and its efficacy was higher than the 50 percent threshold stipulated by the World Health Organization.
Malaysian state-run pharmaceutical company Pharmaniaga on Jan. 12 reached a deal on COVID-19 vaccine cooperation with Sinovac. The Chinese firm will provide 14 million doses of ready-to-fill COVID-19 vaccines to Malaysia, and the Malaysian company will carry out a fill-and-finish process of the vaccine in Malaysia.
The Ukrainian Lekhim Group also partnered with Sinovac on the purchase of 5 million doses of CoronaVac vaccine. Besides, the Ukrainian firm is planning to produce locally multiple Sinovac vaccines, including the CoronaVac, from 2022.
Brazilian Health Minister Eduardo Pazuello said on Jan. 7 that Brazil has signed an agreement with the Butantan Institute of Sao Paulo to purchase 100 million doses of CoronaVac vaccine.
The Department of Health of the Philippines recently disclosed that the Philippine government has secured 25 million doses of Sinovac-developed CoronaVac vaccine. The country's Food and Drug Administration said it has received an application from the Chinese firm seeking an emergency use authorization for the CoronaVac vaccine. The vaccine is expected to be granted as early as this February.
 Menu
Menu
 China's CoronaVac vaccine recognized by multiple countries
China's CoronaVac vaccine recognized by multiple countries