By Qiu Chaoyi, People’s Daily

Employees of Hubei Ourselves Garment Co., Ltd., Qianjiang, Hubei province manufacture protective suits, March 25, 2020. The company can produce 8,000 protective suits per day which are exported to the U.S., Japan, South Korea and the European Union. Photo by Wu Yanjun/ People’s Daily Online
“We will spare no effort to ensure the quality of exports, set standards for businesses to operate with and safeguard the reputation of "Made in China," so as to better leverage the important role of medical supplies in preventing and controlling the pandemic,” said Jiang Fan, first-level inspector of the Department of Foreign Trade at the Ministry of Commerce (MOFCOM) at a press conference on strengthening quality control for medical supplies and improving market regulation on April 5.
Jiang introduced that the MOFCOM has worked with the General Administration of Customs (GAC) and the National Medical Products Administration (NMPA) to issue an announcement on the orderly export of medical supplies.
The announcement requires five categories of exports — including test kits, medical masks, medical protective clothing, ventilators and infrared thermometers — to be officially registered in China and meet the quality-control standards of respective export destinations. The customs shall grant access to those medical supplies based on certificates of registration approved by medical product administrations.
Jin Hai, chief of the Department of General Operation at the GAC, noted that the orderly exportation of medical materials becomes a critical measure to deepen international cooperation on pandemic prevention and control and to jointly tackle the global public health crisis.
Statistics indicated that from March 1 to April 4, Customs inspected and released main epidemic prevention and control materials with a total value of 10.2 billion yuan($1.43 billion), including 3.86 billion face masks, 37.52 million sets of protective suits, 2.41 million infrared thermometers and 16,000 ventilators, with their value totaling 7.72 billion yuan, 910 million yuan, 330 million yuan and 310 million yuan, respectively. In addition, there were also 2.84 million COVID-19 testing kits and 8.41 million goggles. From the perspective of trade mode, the general trade accounted for 83%, with the value totaling 8.52 billion yuan.
A working group was established by the GAC to ensure the smooth clearance of epidemic prevention and control materials. Exporters of medical products including COVID-19 testing kits, medical face masks, medical protective suits, ventilators and infrared thermometers have to provide extra documentation when these goods go through customs.
Enterprises are encouraged to offer electronic statements. The GAC will strengthen the customs protection of intellectual property rights, and resolutely punish infringements on pandemic prevention products. It will also crack down on all the illegal acts in accordance with the law, such as export fraud and concealment, secretly carrying, passing off fake goods as genuine ones and unqualified goods pretending to be qualified ones.
At present, local market regulation departments are launching special rectification campaigns, focusing on such illegal activities as forging, falsely using, buying and selling authentication certificates, conducting certification activities without permission or in a non-standard way, and illegal pricing in the certification activities, said Liu Weijun, head of the Department of Certification Regulation at the State Administration for Market Regulation.
By the end of March, a total of 8,069 inspections in the form of emergency registration inspections, emergency evaluation inspections and supervised sampling inspections had been conducted on medical devices for epidemic prevention and control, according to Zhang Qi, deputy head of the Department of Medical Device Regulation at the National Medical Products Administration (NMPA). The NMPA and the State Administration for Market Regulation (SAMR) have dispatched several competent law enforcement officers to 14 key provinces and municipalities, including Beijing, Tianjin, Zhejiang, Hubei and Guangdong to supervise and inspect the local quality control of medical devices for epidemic prevention and control.
Jiang said that as of April 4, 54 countries and regions, as well as three international organizations, had signed commercial procurement contracts with Chinese enterprises for medical supplies. Moreover, 74 countries and 10 international organizations are conducting procurement negotiations with Chinese companies.
Jiang introduced that the MOFCOM has worked with the General Administration of Customs (GAC) and the National Medical Products Administration (NMPA) to issue an announcement on the orderly export of medical supplies.
The announcement requires five categories of exports — including test kits, medical masks, medical protective clothing, ventilators and infrared thermometers — to be officially registered in China and meet the quality-control standards of respective export destinations. The customs shall grant access to those medical supplies based on certificates of registration approved by medical product administrations.
Jin Hai, chief of the Department of General Operation at the GAC, noted that the orderly exportation of medical materials becomes a critical measure to deepen international cooperation on pandemic prevention and control and to jointly tackle the global public health crisis.
Statistics indicated that from March 1 to April 4, Customs inspected and released main epidemic prevention and control materials with a total value of 10.2 billion yuan($1.43 billion), including 3.86 billion face masks, 37.52 million sets of protective suits, 2.41 million infrared thermometers and 16,000 ventilators, with their value totaling 7.72 billion yuan, 910 million yuan, 330 million yuan and 310 million yuan, respectively. In addition, there were also 2.84 million COVID-19 testing kits and 8.41 million goggles. From the perspective of trade mode, the general trade accounted for 83%, with the value totaling 8.52 billion yuan.
A working group was established by the GAC to ensure the smooth clearance of epidemic prevention and control materials. Exporters of medical products including COVID-19 testing kits, medical face masks, medical protective suits, ventilators and infrared thermometers have to provide extra documentation when these goods go through customs.
Enterprises are encouraged to offer electronic statements. The GAC will strengthen the customs protection of intellectual property rights, and resolutely punish infringements on pandemic prevention products. It will also crack down on all the illegal acts in accordance with the law, such as export fraud and concealment, secretly carrying, passing off fake goods as genuine ones and unqualified goods pretending to be qualified ones.
At present, local market regulation departments are launching special rectification campaigns, focusing on such illegal activities as forging, falsely using, buying and selling authentication certificates, conducting certification activities without permission or in a non-standard way, and illegal pricing in the certification activities, said Liu Weijun, head of the Department of Certification Regulation at the State Administration for Market Regulation.
By the end of March, a total of 8,069 inspections in the form of emergency registration inspections, emergency evaluation inspections and supervised sampling inspections had been conducted on medical devices for epidemic prevention and control, according to Zhang Qi, deputy head of the Department of Medical Device Regulation at the National Medical Products Administration (NMPA). The NMPA and the State Administration for Market Regulation (SAMR) have dispatched several competent law enforcement officers to 14 key provinces and municipalities, including Beijing, Tianjin, Zhejiang, Hubei and Guangdong to supervise and inspect the local quality control of medical devices for epidemic prevention and control.
Jiang said that as of April 4, 54 countries and regions, as well as three international organizations, had signed commercial procurement contracts with Chinese enterprises for medical supplies. Moreover, 74 countries and 10 international organizations are conducting procurement negotiations with Chinese companies.
 Menu
Menu
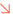 China takes tough measures to ensure export quality of pandemic prevention materials
China takes tough measures to ensure export quality of pandemic prevention materials
















