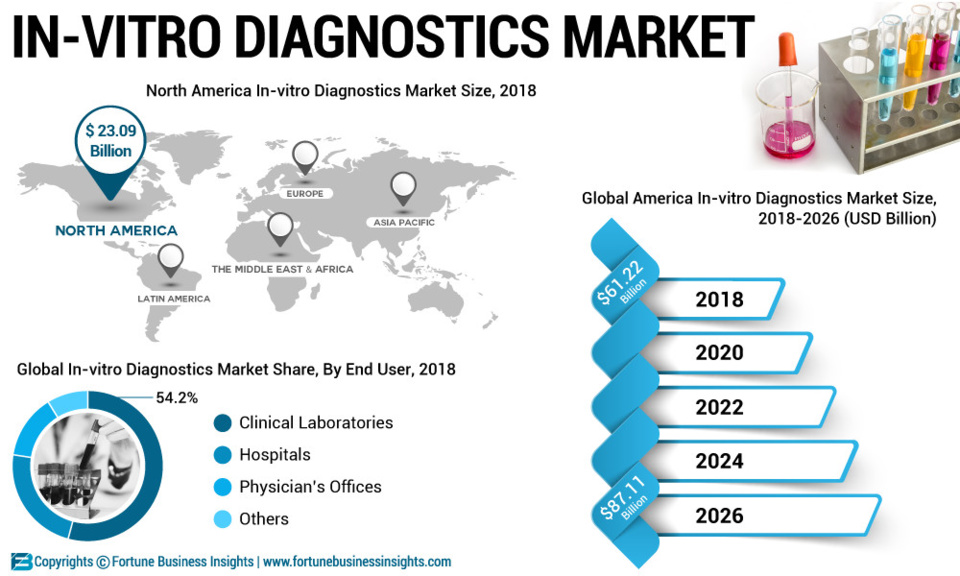
“Increasing Prevalence of Chronic and Infectious Disease Will Enable Growth “
The launch of diagnostic and rapid testing tools by leading market players will propel growth of the global in-vitrodiagnostics market. For instance, Thermo Fisher Scientific launched Phadia 200 for diagnosis of allergy & autoimmune conditions. The launch of Phadia 200 is anticipated to increase the revenue of the company. According to the report, the reagents and consumables segment will account for a major portion in the global in-vitro diagnostics market during the forecast period owing to the adoption of self- test and point-of-care devices. Furthermore, the instrument segment is likely to grow at a moderately slower pace during the forecast period. Rising technological advancement is predicted to aid growth of the segment. Moreover, the increasing cases of cancer and infectious disease around the world will further accelerate global in-vitro diagnostics market growth.
“Launch of Altostar Molecular Diagnostics Workflow Will Boost Growth”
Altona Diagnostics GmbH, molecular diagnostic testing solutions company launched a CE-IVD marked AltoStar Molecular Diagnostics Workflow. AltoStar Molecular Diagnostics a flexible and efficient automatic system that automates the entire workflow from sample preparation up to analysis. Fortune Business Insights states the launch of CE-IVD marked AltoStar Molecular Diagnostics Workflow is expected to boost the in-vitro diagnostics market revenue. Furthermore, the oncology segment is likely to grow at a considerable rate during the forecast period. Rising adoption and availability of advanced home care kits such as fecal occult blood test (FOBT) for diagnosis of colon cancer in homecare settings is one of the major factor likely to fuel demand for the oncology segment, which, will, in turn, uplift the global in-vitro diagnostics market shares
Source: https://www.fortunebusinessinsights.com/industry-reports/in-vitro-diagnostics-ivd-market-101443
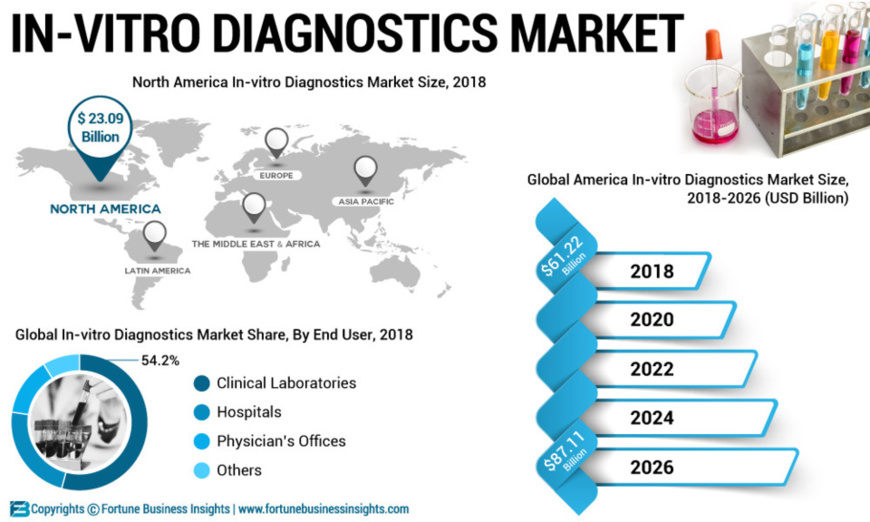
The launch of diagnostic and rapid testing tools by leading market players will propel growth of the global in-vitrodiagnostics market. For instance, Thermo Fisher Scientific launched Phadia 200 for diagnosis of allergy & autoimmune conditions. The launch of Phadia 200 is anticipated to increase the revenue of the company. According to the report, the reagents and consumables segment will account for a major portion in the global in-vitro diagnostics market during the forecast period owing to the adoption of self- test and point-of-care devices. Furthermore, the instrument segment is likely to grow at a moderately slower pace during the forecast period. Rising technological advancement is predicted to aid growth of the segment. Moreover, the increasing cases of cancer and infectious disease around the world will further accelerate global in-vitro diagnostics market growth.
“Launch of Altostar Molecular Diagnostics Workflow Will Boost Growth”
Altona Diagnostics GmbH, molecular diagnostic testing solutions company launched a CE-IVD marked AltoStar Molecular Diagnostics Workflow. AltoStar Molecular Diagnostics a flexible and efficient automatic system that automates the entire workflow from sample preparation up to analysis. Fortune Business Insights states the launch of CE-IVD marked AltoStar Molecular Diagnostics Workflow is expected to boost the in-vitro diagnostics market revenue. Furthermore, the oncology segment is likely to grow at a considerable rate during the forecast period. Rising adoption and availability of advanced home care kits such as fecal occult blood test (FOBT) for diagnosis of colon cancer in homecare settings is one of the major factor likely to fuel demand for the oncology segment, which, will, in turn, uplift the global in-vitro diagnostics market shares
Source: https://www.fortunebusinessinsights.com/industry-reports/in-vitro-diagnostics-ivd-market-101443
 Menu
Menu
 n-Vitro Diagnostics Market To Reach USD 87.11 Billion By 2026; Increasing Prevalence of Cancer Will Enable Growth
n-Vitro Diagnostics Market To Reach USD 87.11 Billion By 2026; Increasing Prevalence of Cancer Will Enable Growth